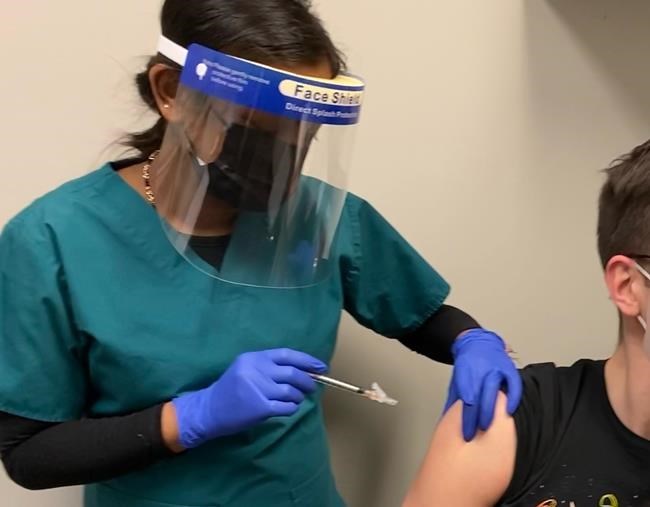
CALGARY — Human clinical trials have begun in Toronto for a proposed COVID-19 vaccine made by a Canadian company.
Providence Therapeutics of Calgary says 60 subjects will be monitored for 13 months, with the first results expected next month.
The group of healthy volunteers aged 18 to 65 have been divided into four groups of 15. Three of the groups will get three different dose levels, while a fourth group gets a placebo.
Pending regulatory approval, the company's CEO Brad Sorenson says a larger Phase 2 trial may start in May with seniors, younger subjects and pregnant people.
Providence uses messenger RNA technology for a product it calls PTX-COVID19-B.
Sorenson says if successful, the vaccine could be released by the end of the year.
“We are thrilled to begin human clinical trials of PTX-COVID19-B. Having a made-in-Canada solution to address the global COVID-19 pandemic will augment the reliability of vaccine supply for Canadians, contribute to the global vaccine supply and position a Canadian company on the global stage as a contributor to the solution,” Sorenson said Tuesday in a release.
This report by The Canadian Press was first published Jan. 26, 2021.
The Canadian Press